Publications
Independent Publications
*denotes co-first authors
2025
Sahu, S.*; Paikin, Z. E.*; Talbott, J. M.; Czabala, P.; Raj, M. Coarctate reaction for synthesis of fluorescent N-heterocycles, late-stage functionalization, and photo-triggered drug delivery. Nat. Commun. 2025, 16, 3780. (pdf)
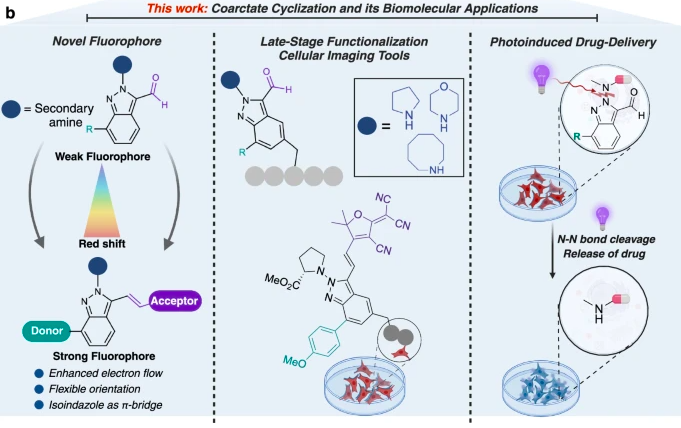
Talbott, J. M.*; Wills, R.*; Shirke, R.*; Hassanein, L.; Weinshenker, D.; Raj, M. Spatiotemporal Imaging of Catechol Aldehydes in Neural Tissue. JACS Au 2025, 5, 1717-1727. (pdf)
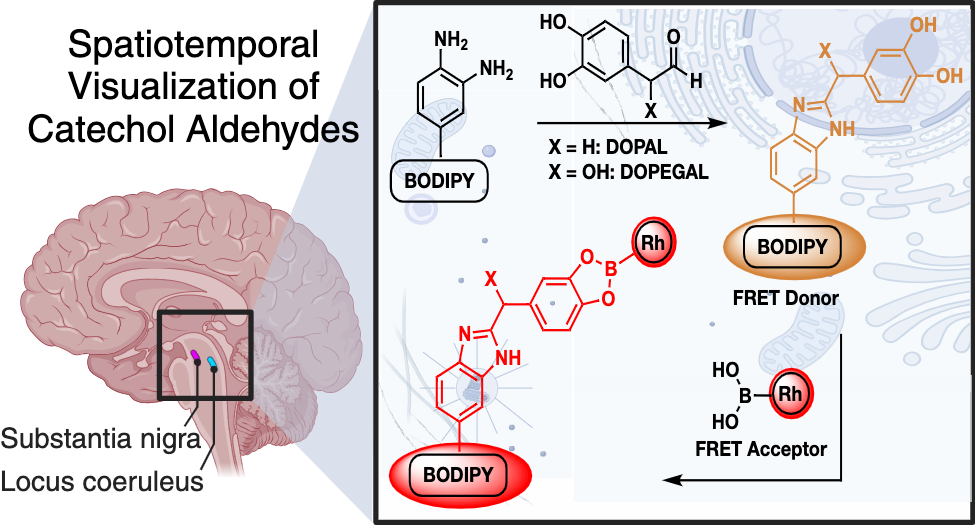
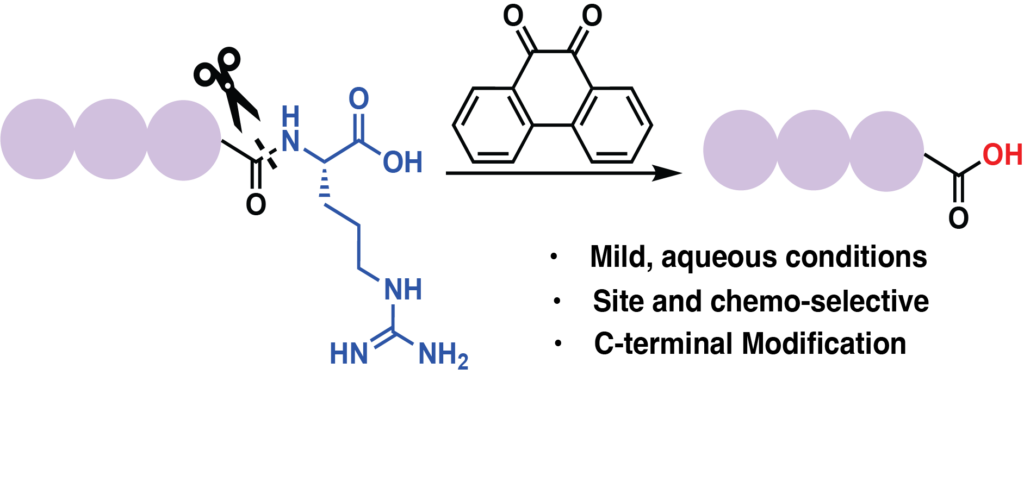
Prosser, L. P.*; Emenike, B.*; Sihag, P.*; Raj, M. Chemical Carbonylation of Arginine in Peptides and Proteins. J. Am. Chem. Soc. 2025, 147, 10139-10150. (pdf)
Paikin, Z. E.*; Villalobos Galindo, A.*; Raj, M. Metabolite Mediated Protein Macrocyclization. Synlett. 2025, 36, 1379-1384. (pdf)
Farhi, J.*; Emenike, B.*; Lee, R. S.; Sad, K.; Fawwal, D. V.; Beusch, C. M.; Jones, R. B.; Verma, A. K.; Jones, C. Y.; Foroozoni, M.; Reeves, M.; Parwani, K. K.; Bagchi, P.; Deal, R. B.; Katz, D. J.; Corbett, A. H.; Gordon, D. E.; Raj, M.; Spangle, J. M. Dynamic In Vivo Mapping of the Methylproteome Using a Chemoenzymatic Approach. J. Am. Chem. Soc. 2025, 147, 7214-7230. (pdf)
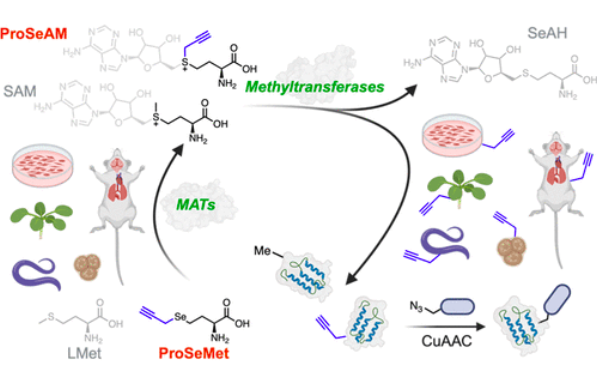
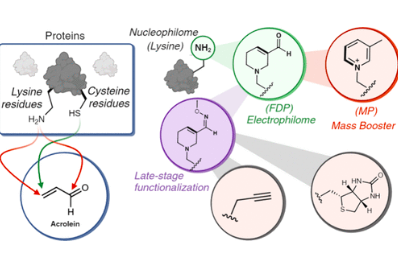
Paikin, Z. E.*; Emenike, B.*; Shirke, R.; Beusch, C. M.; Gordon, D. E.; Raj, M. Acrolein-Mediated Conversion of Lysine to Electrophilic Heterocycles for Protein Diversification and Toxicity Profiling. J. Am. Chem. Soc. 2025, 147, 5692. (pdf)
2024
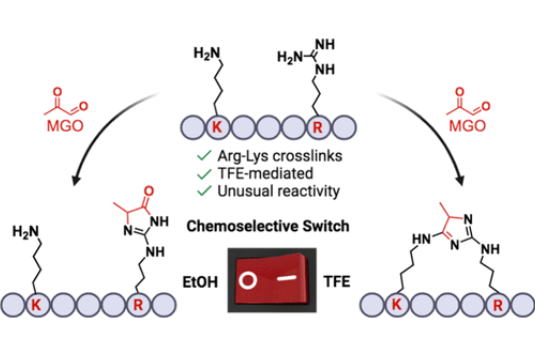
Villalobos Galindo, A.; Raj, M. Solvent-Dependent Chemoselectivity Switch to Arg-Lys Imidazole Cross-Links. Org. Lett. 2024, 26, 8356-8360. (pdf)
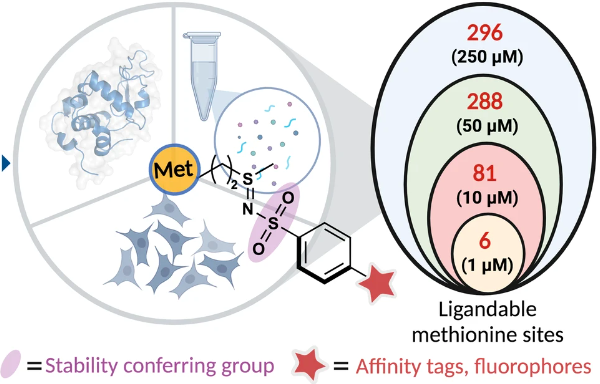
Sahu, S.*; Emenike, B.*; Buesch, C. M.; Bagchi, P.; Gordon, D. E.; Raj, M. Copper(I)-nitrene platform for chemoproteomic profiling of methionine. Nat. Commun. 2024, 15, 4243. (pdf)
Bruce, A.*; Adebomi, V.*; Czabala, P.; Palmer, J.; McFadden, W. M.; Lorson, Z. C.; Slack, R. L.; Bhardway, G.; Sarafianos, S. G.; Raj, M. A Tag-Free Platform for Synthesis and Screening of Cyclic Peptide Libraries. Angew. Chem., Int. Ed. 2024, 63, e202320045. (pdf)
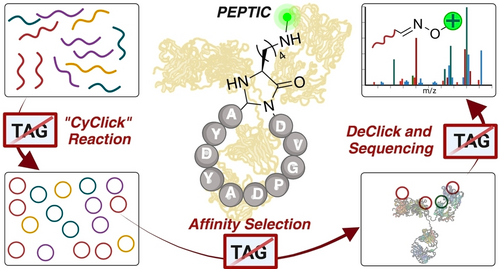
Emenike, B.; Czabala, P.; Farhi, J.; Swaminathan, J.; Anslyn, E. V.; Spangle, J.; Raj, M. Tertiary Amine Coupling by Oxidation for Selective Labelling of Dimethyl Lysine Post-Translational Modifications. J. Am. Chem. Soc. 2024, 146, 10621-10631. (pdf)
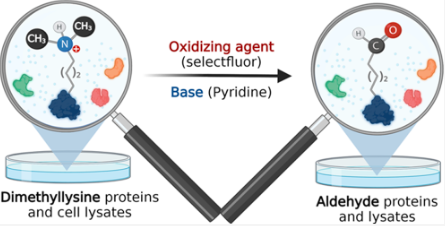
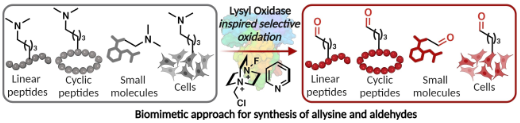
Emenike, B.*; Shahin, S.*; Raj, M. Bioinspired Synthesis of Allysine for Late-Stage Functionalization of Peptides. Angew. Chem., Int. Ed. 2024 e202403215. (pdf)
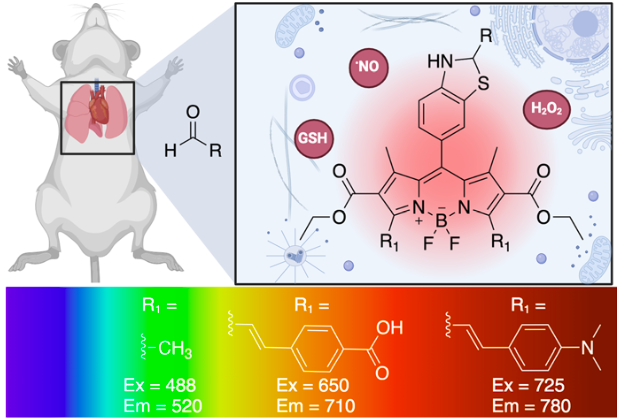
Wills, R.*; Shirke, R.*; Hrncir, H.; Talbott, J. M.; Sad, K.; Spangle, J. M.; Gracz, A. D.; Raj, M. Tunable fluorescent probes for detecting aldehydes in living systems. Chem. Sci. 2024, 15, 4673-4769. (pdf) Selected for Cover Image
Selected to 2024 Most Popular Analytical Articles
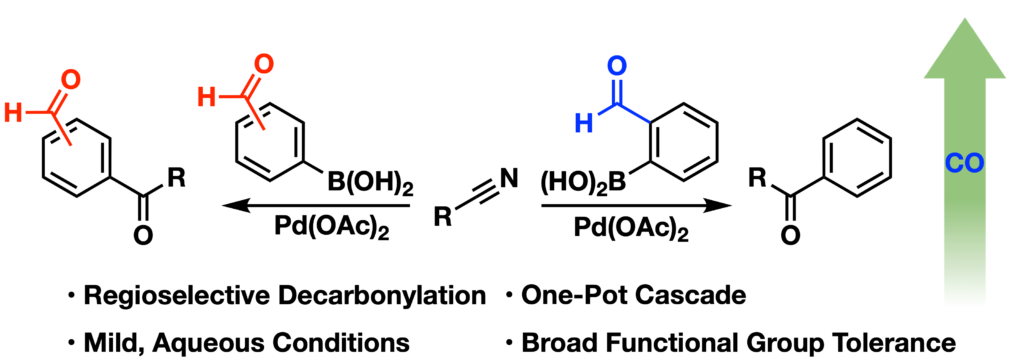
Paikin, Z. E.; Talbott, J. M.; Raj, M. Regioselective Aldehyde Decarbonylation through Palladium-Catalyzed Nitrile Boronic Acid Cross-Coupling. Synlett 2024, 35, 1924-1928. (pdf)
2023
Prosser, L. P.*; Talbott, J. M.*; Garrity, R. P.; Raj, M. C-Terminal Arginine-Selective Cleavage of Peptides as a method for Mimicking Carboxypeptidase B. Org. Lett. 2023, 25, 6206-6210. (pdf)
Most viewed Org. Lett. article of August 2023
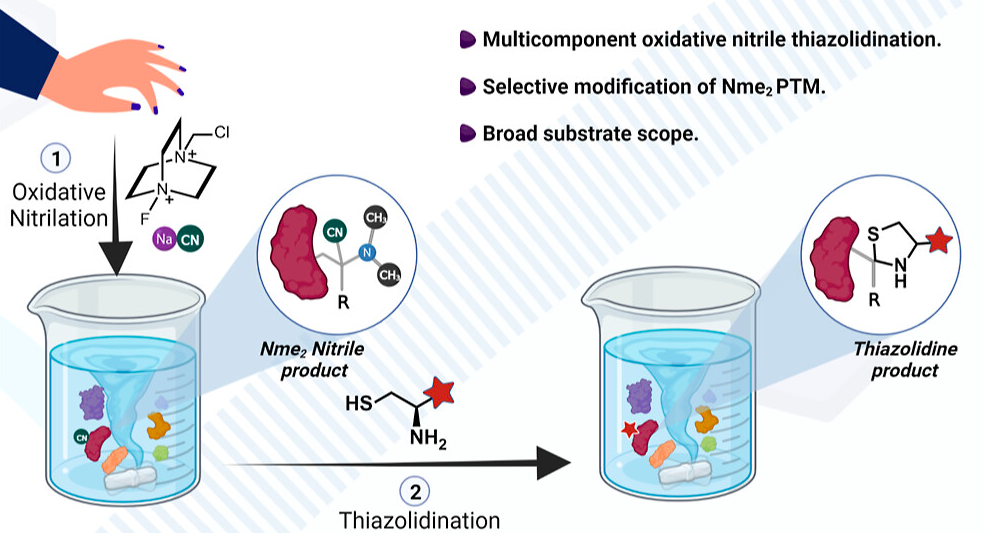
Emenike, B.; Donovan, J.; Raj, M. Multicomponent Oxidative Nitrile Thiazolidination Reaction for Selective Modification of N-terminal Dimethylation Posttranslational Modification. J. Am. Chem. Soc. 2023, 154, 16417-16428. (pdf)
Wills, R.; Farhi, J.; Czabala, P.; Shahin, S.; Spangle, J. M.; Raj, M. Chemical sensors for imaging total cellular aliphatic aldehydes in live cells. Chem. Sci. 2023, 14, 8305-8314. (pdf)
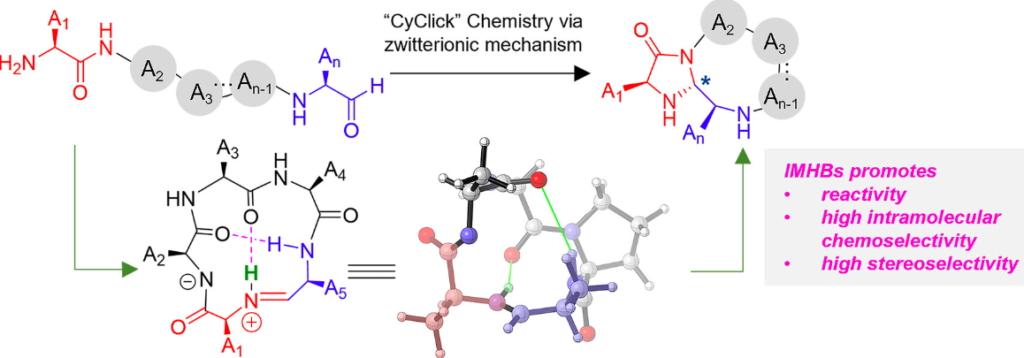
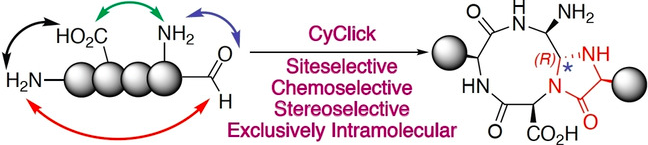
Shao, H.; Adebomi, V.; Bruce, A.; Raj, M.; Houk, K. N. Intramolecular Hydrogen Bonding Enables a Zwitterionic Mechanism for Macrocyclic Peptide Formation: Computational Mechanistic Studies of CyClick Chemistry. Angew. Chem., Int. Ed. 2023, e202307210. (pdf)
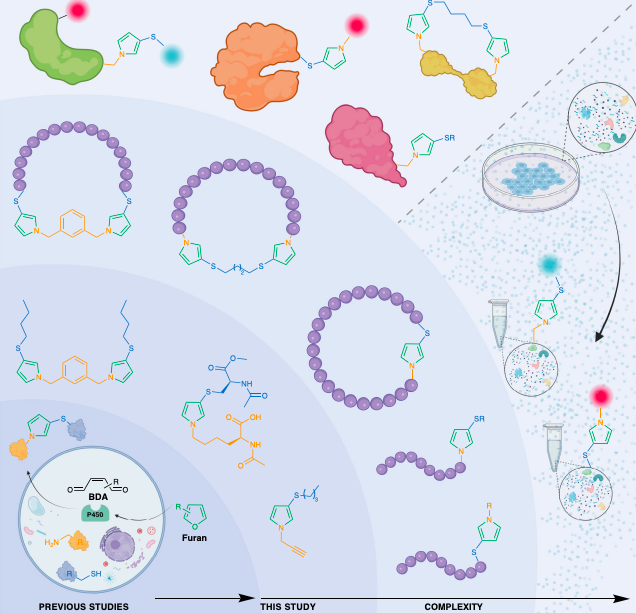
Wang, Y.; Czabala, P.; Raj, M. Bioinspired one-pot furan-thiol-amine multicomponent reaction for making heterocycles and its applications. Nat. Commun. 2023, 14, 4086-4101. (pdf)
2022
Emenike, B.; Nwajiobi, O.; Raj, M. Covalent chemical tools for profiling post-translational modifications. Front. Chem. 2022, 10, 868773. (pdf)
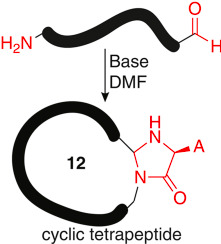
Wills, R.; Adebomi, V.; Spancake C.; Raj, M. Synthesis of L-Cyclic Tetrapeptides by Backbone Amide Activation CyClick Strategy. Tetrahedron 2022, 126, 133071. (pdf)
Adebomi, V.; Wang, Y.; Sriram M.; Raj, M. Selective Conversion of Unactivated C-N Amide bond to C-C bond via Steric and Electronic Resonance Destabilization Under Metal-Free Conditions. Org. Lett. 2022, 24, 6525-6530. (pdf)
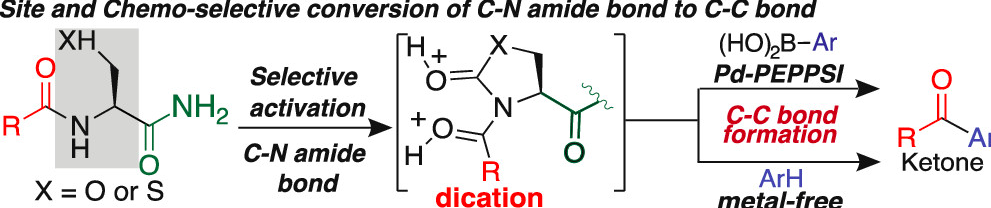
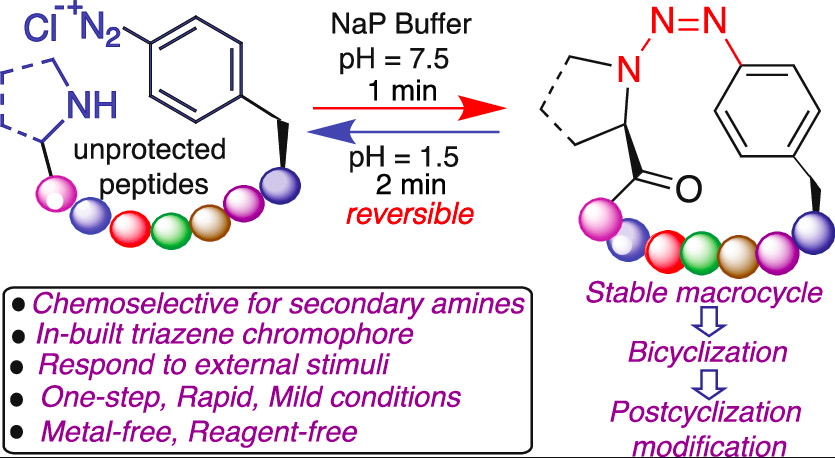
Nwajiobi, O.; Verma, A.; Raj, M. Rapid Arene Triazene Chemistry for Macrocyclization. J. Am. Chem. Soc. 2022, 144, 4633-4641. (pdf)
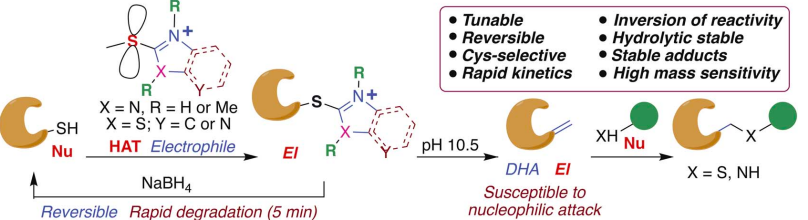
Tang, K.-C.; Maddox, S.; Backus, K. M.; Raj, M. Tunable Heteroaromatic Azoline thioethers (HAT) for Cysteine Selective Modification and Proteome profiling. Chem. Sci. 2022, 13, 763-774. (pdf)
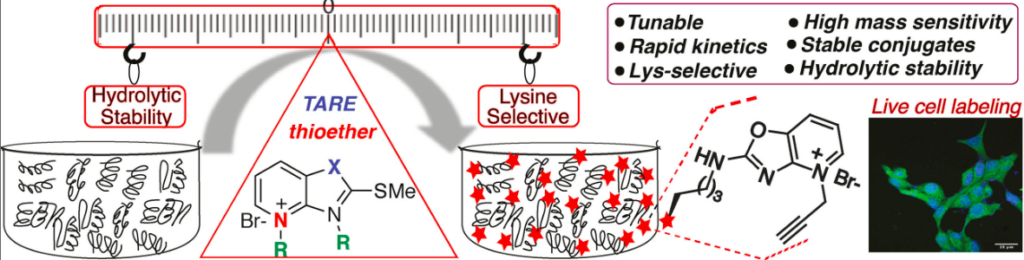
Tang, K.-C.; Cao, J.; Boatner, L. M.; Li, L.; Farhi, J.; Houk, K. N.; Spangle, J.; Backus, K. M.; Raj, M. Tunable Amine-Reactive Electrophiles for Selective Profiling of Lysine. Angew. Chem., Int. Ed. 2022, 61, e202112107. (pdf)
2021
Adebomi, V.; Sriram, M.; Streety, X.; Raj, M. Metal-Free Selective Modification of Secondary Amides: Application in Late-Stage Diversification of Peptides. Org. Lett. 2021, 23, 6189–6193. (pdf)
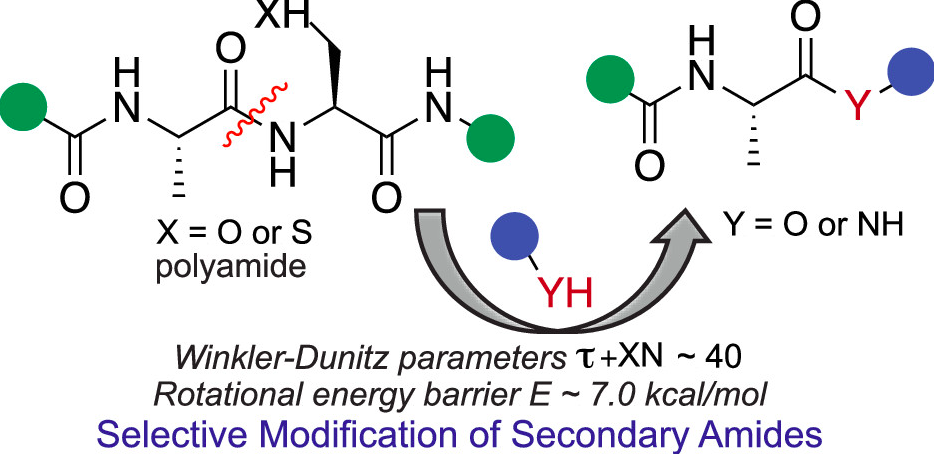
Nwajiobi, O.; Sriram, M.; Streety, X.; Raj, M. Selective Triazenation Reaction (STaR) of Secondary Amines for Tagging Monomethyl Lysine Post-Translational Modifications. Angew. Chem., Int. Ed. 2021, 60, 7344-7352. (pdf)
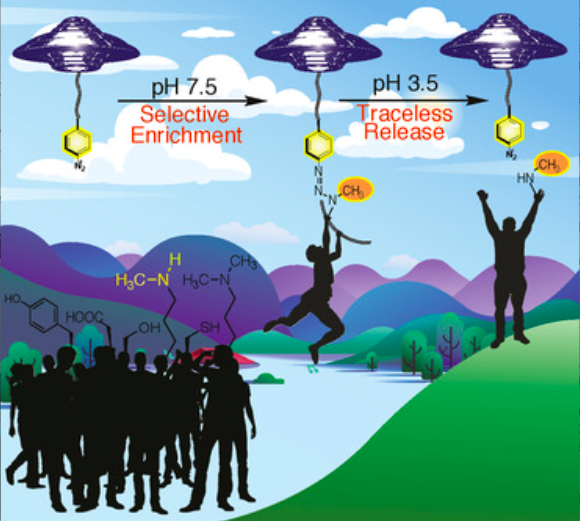
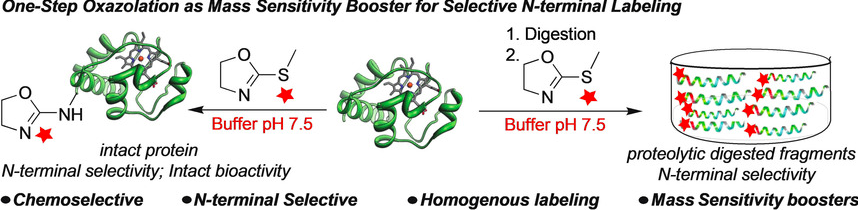
Tang, K.-C.; Raj, M. One step azolation strategy for site- and chemo-selective labeling of proteins with mass sensitive probes. Angew. Chem., Int. Ed. 2021, 60, 1797-1805. (pdf)
2020
Wills, R.; Adebomi, V.; Raj, M. Site selective peptide macrocyclization. ChemBioChem. 2020, 22, 52-62. (Invited Review article) (pdf)
Wills, R.; Adebomi, V.; Raj, M. Peptide cyclization at high concentration. Synlett 2020, 31, 1537-1542. (Invited Review article) (pdf)
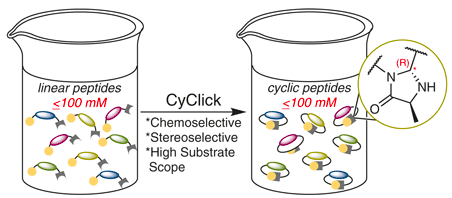
Sriram, M.; Adebomi, V.; Raj, M. Bioinspired nitroalkylation for selective protein modification and peptide stapling. Angew. Chem., Int. Ed. 2020, 59, 2793-2801. (pdf)
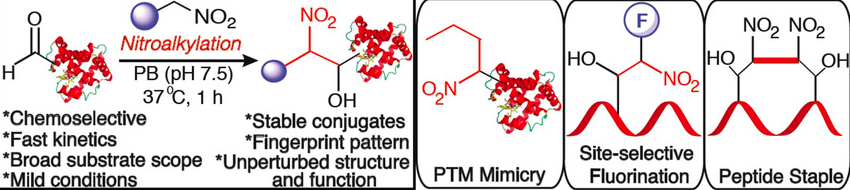
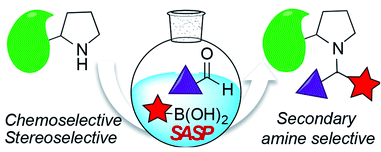
Sim, Y. E.; Nwajiobi, O.; Sriram, M.; Cohen, R. D.; Raj, M. Secondary amine selective peptide (SASP) bioconjugation. Chem. Sci. 2020, 11, 53-61. (pdf)
2019
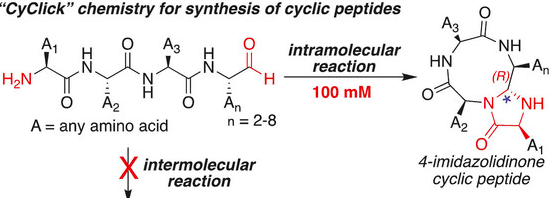
Adebomi, V.; Cohen, R. D.; Wills, R.; Chavers, H. A. H.; Martin, G. E.; Raj, M. CyClick chemistry for synthesis of cyclic peptides. Angew. Chem., Int. Ed. 2019, 58, 19073-19080. (pdf)
2018
Sriram, M.; Tang, K.-C.; Raj, M. Amide bond activation of biological molecules. Molecules 2018, 23, 2615. (Invited Review article) (pdf)
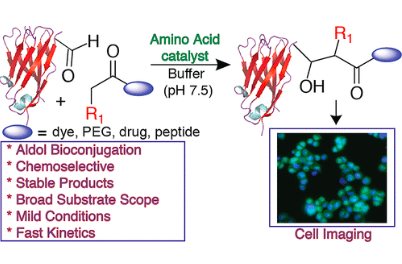
Howard, T. S.; Cohen, R. D.; Nwajiobi, O.; Muneeswaran, Z. P.; Sim, Y. E.; Lahankar, N. N.; Yeh, J.; Raj, M. Amino-acid-catalyzed direct aldol bioconjugation. Org. Lett. 2018, 20, 5344-5347. (pdf)
Elashal, H. E.; Cohen, R. D.; Elashal, H. E.; Zong, C.; Link, A. J.; Raj, M. Cyclic and lasso peptides: sequence determination, topology analysis, and rotaxane formation. Angew. Chem., Int. Ed. 2018, 57, 6150-6154. (pdf)
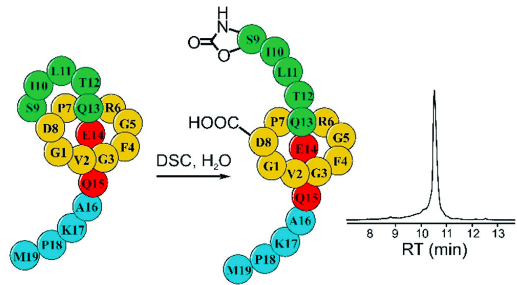
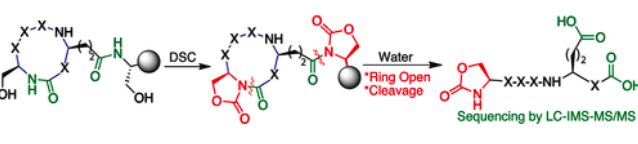
Elashal, H. E.; Cohen, R. D.; Elashal, H. E.; Raj, M. Oxazolidinone-mediated sequence determination of one-bead one-compound cyclic peptide libraries. Org. Lett. 2018, 20, 2374-2377. (pdf)
Zong, C.; Ling, W.; Lee, C.; Elashal, H. E.; Raj, M.; Link, A. J. Albusnodin: an acetylated lasso peptide from Streptomyces albus. Chem. Commun. 2018, 54, 1339-1342. (pdf)
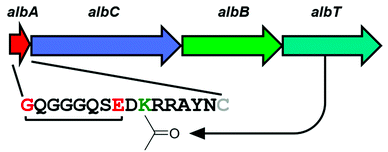
2017
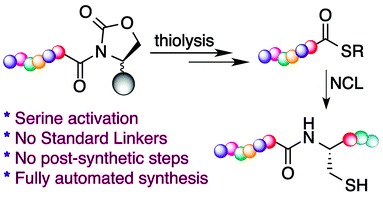
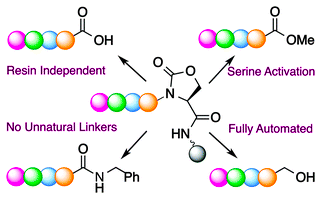
Elashal, H. E.; Sim, Y. E.; Raj, M. Serine promoted synthesis of peptide thioester-precursor on solid support for native chemical ligation. Chem. Sci. 2017, 8, 117-123. (pdf)
2016
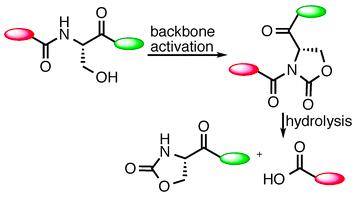
Elashal, H. E.; Cohen, R. D.; Raj, M. Fmoc solid-phase synthesis of C-terminal modified peptides by formation of a backbone cyclic urethane moiety. Chem. Commun. 2016, 52, 9699-9702. (pdf)
Elashal, H. E.; Raj, M. Site-selective chemical cleavage of peptide bonds. Chem. Commun. 2016, 52, 6304-6307. (pdf)
Nalbone, J. M.; Lahankar, N.; Buissereth, L.; Raj, M. Glutamic acid selective chemical cleavage of peptide bonds. Org. Lett. 2016, 18, 1186–1189. (pdf)
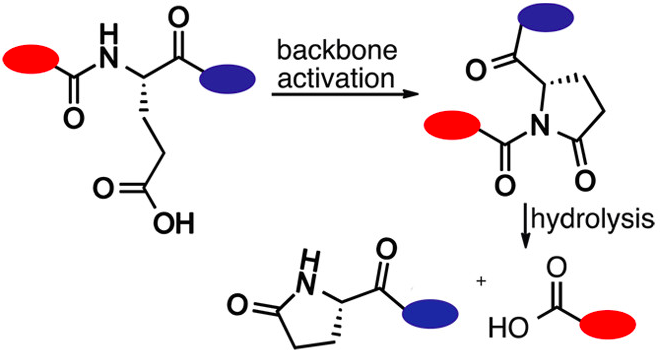
Ph.D. and Post Doc. Publications
Wu, H.; Handoko; Raj, M.; Arora, P. S. Iterative design of a biomimetic catalyst for amino acid thioester condensation. Org. Lett. 2017, 19, 5122-5125. (pdf)
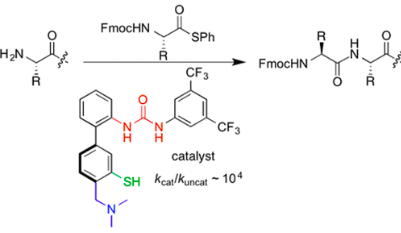
Raj, M.; Wu, H.; Blosser, S.; Vittoria, M.; Arora, P. S. Aldehyde capture ligation for synthesis of native peptide bonds. J. Am. Chem. Soc. 2015, 37, 6932-6940. (pdf)
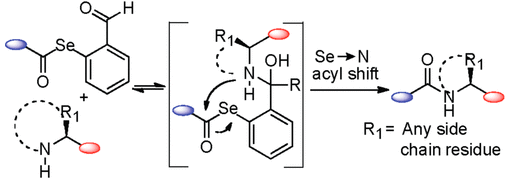
Raj, M.; Bullock, B. N.; Arora, P. S. Plucking the high hanging fruit: A systematic approach for targeting protein-protein interactions. Bioorg. Med. Chem. 2013, 21, 4051-4057. (pdf)
Kaur, J.; Raj, M.; Cooperman, B. S. Fluorescent labeling of tRNA dihydrouridine residues: mechanism and distribution. RNA, 2011, 17, 1393-1400. (pdf)
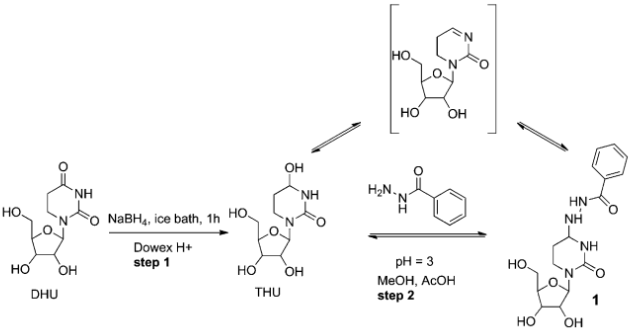
Raj, M.; Veerasamy, N.; Singh, V. K. Highly enantioselective synthesis of 3-cycloalkanone-3-hydroxy-2-oxindoles: potential anticonvulsants. Tetrahedron Lett. 2010, 51, 2157-2159. (pdf)
Raj, M.; Singh, V. K. Organocatalytic reactions in water. Chem. Commun. 2009, 44, 6687-6703. (pdf)
Raj, M.; Parashari, G. S.; Singh, V. K. Direct syn- and anti-aldol reaction catalyzed by primarytertiary diamines-Brønsted acids in aqueous medium. Adv. Synth. Catal. 2009, 351, 1284-1288. (pdf)
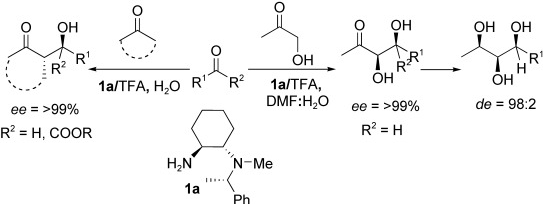
Raj, M.; Maya, V.; Singh, V. K. Highly efficient small organic molecules for enantioselective direct aldol reaction in both organic & aqueous medium: application in synthesis. J. Org. Chem. 2009, 74, 4289-4297. (pdf)
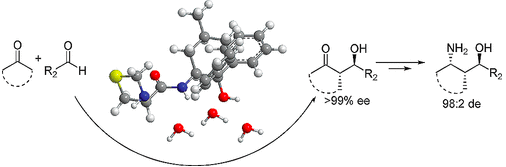
Vishnumaya, Raj, M.; Singh, V. K. Highly enantioselective organocatalytic direct aldol reaction in an aqueous medium. Org. Lett, 2007, 9, 2593-2595. (pdf)
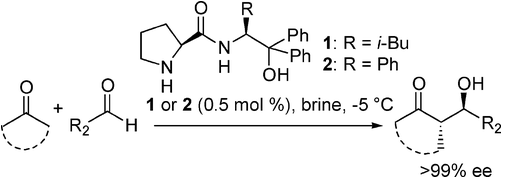
Raj, M.; Vishnumaya;, Ginotra, S. K.; Singh, V. K. “Highly enantioselective direct aldol reaction catalyzed by organic molecules” Org. Lett. 2006, 8, 4097-4099. (pdf) Most accessed article in 2006. Highlighted in Synfacts: Synfacts 2006(10): 1066-1066.
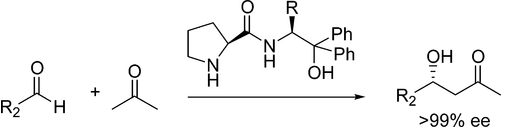
Raj, M.; Vishnumaya; Ginotra, S. K.; Singh, V. K. Org. Lett. 2006, 8, 4097-4099. Commercialization of my two organocatalysts by SigmaAldrich http://www.sigmaaldrich.com/catalog/papers/16928083
Book Chapters and Patents
“Catalytic Methods in Asymmetric Synthesis: Advanced Materials, Techniques, and Applications” Book Chapter (Monika Raj and Vinod K. Singh 2010 published by John Wiley and Sons).
Patents
K. Tang, B. Emenike, and M. Raj “Compostions and Methods for Selective Labeling of Primary Amides with Iodovinyl Compounds” United States Patent Application No. 63/398,645; Emory Tech ID 22175
B. Emenike, J. Talbott, and M. Raj “Redox-Neutral Bioconjugation Reaction for Asparagine and Glutamine” United States Patent Application No. 63/442,227; Emory Tech ID 23081
P. Rodriguez and M. Raj “Compositions and Methods for Selective Labeling of N-Alkylated Imidazole Containing Compounds and Peptides” United States Patent Application No. 63/400,155; Emory Tech ID 21199
K. Tang and M. Raj “Selective Tagging of Monomethyl Lysine” International Patent Application No. PCT/US2022/081743; Emory Tech ID 21195
M. Raj “Selective Tagging of Serine and Threonine” United States Patent Application No. 63/443,743; Emory Tech ID 23090
V. Adebomi and M. Raj “Selective Tagging of Aliphatic Amino Acid (Val-Ile-Lue)” United States Patent Application No. 63/398,634
K. Tang and M. Raj “Compositions and Methods for Selective Labeling of Secondary Amines” United States Patent Application No. 63/290,679
B. Emenike and M. Raj “Compositions and Methods for Labeling of Thioethers” United States Patent Application No. PCT/US2022/081837; Emory Tech ID 21197
B. Emenike and M. Raj “Methods Detecting Tertiary Amines and Compositions Related Thereto” International Patent Application No. PCT/US2022/076782; Emory Tech ID 21187
V. Adebomi and M. Raj “Synthetic Cyclic Peptides and methods of Preparation and use thereof” New Invention No.: 17502506
Y. Sim and M. Raj “Secondary Amine Selective Petasis Reaction” and Provisional Application No.: 62/799,934
S. Masesh and M. Raj “Nitroalkylation for Selective Protein Modification and Peptide Stapling” Provisional New AU IP Invention No.: 63/016,472
M. Raj, H. Wu and P. Arora “Aldehyde Capture Ligation for Synthesis of Native Peptide Bonds” from U.S. Pat. Appl. Publ. (2015), US 14/583,586